Loading...


Liquid Embolic System
Brand
- NeuroSafe Medical Co., Ltd
Model
- Lava TM
Manufacturing Country
- China
Quality Certificates
CE MARKING
ISO 13485
MDR
Specification
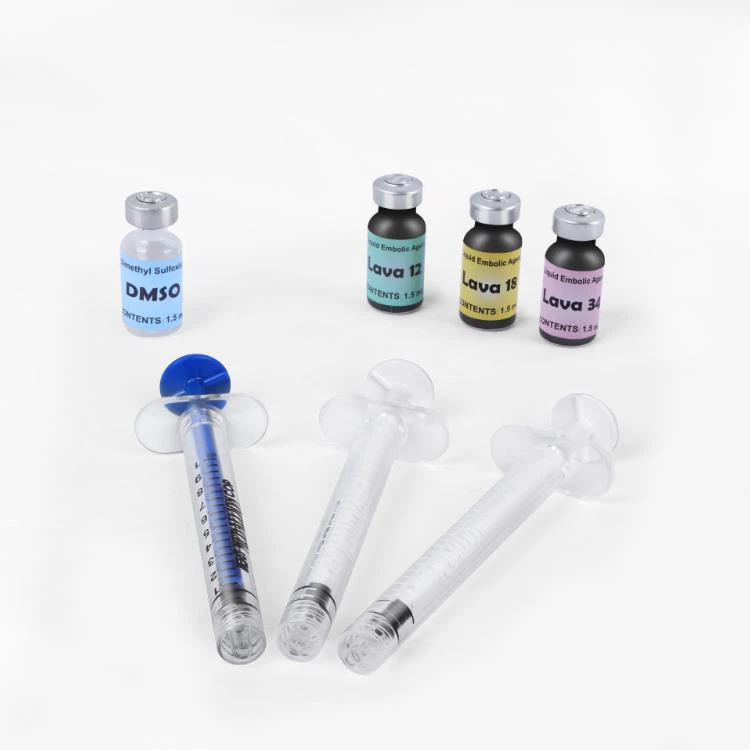
The LavaTM Liquid Embolic System is an interventional endovascular therapy for the treatment of cerebrovascular malformations. Cerebrovascular malformation refers to a group of benign or malignant neurovascular abnormalities that can lead to intracranial hemorrhage, cerebral infarction, cerebral ischemia and other diseases. The liquid embolization system is a medical device administered through simple intravascular insertion, which embolizes abnormal cerebral blood vessels by injecting a special fluid material. The fluid material forms a controlled tissue embolism within blood vessels, which can lessen the impact of cerebrovascular malformations on patients. A DMSO compatible delivery micro catheter that is indicated for use in the neurovasculature is used to access the embolization site. Lava liquid embolic agent is a non‐adhesive liquid embolic agent system comprised of EVOH (ethylene vinyl alcohol) copolymer dissolved in DMSO (dimethyl sulfoxide), and suspended micronized tantalum powder to provide contrast for visualization under fluoroscopy. LavaTM is available in three product formulations, LAVA-12, LAVA-18 and LAVA-34. LAVA-12: Recommended when feeding distal micro vessels and through small feeders. LAVA-18: Recommended when feeding pedicle injections will be conducted close to the nidus; LAVA-34: Recommended for embolizing higher flow and larger fistulous components.
Features&Advantages
1. Non-adhesive, latest technology of EVOH co-polymer.
2. Comprehensive degrees of viscosity (12 cSt, 18 cSt & 34 cSt), accommodate to various clinical requirements.
3. Enhanced fluidness, deeper penetration into the nidus and distal microvessels.
4. Stable and controlled delivery to reach complete occlusion.
5. Micronized grain size of Tantalum powder provides excellent visibility.
Each Lava Kit Contains:
One 1.5 ml vial of Lava-18
A 1.5 ml vial of DMSO
One 1 ml blue syringe for DMSO
Two 1 ml white syringes for Lava
Similar Products
You might also be interested in these products