Loading...

Antibiotic susceptibility testing (AST) panels
Brand
- Erba Mannheim ( Erba Lachema )
Model
- MIKROLATEST® BP
Manufacturing Country
- Czechia
Quality Certificates
CE MARKING
ISO 13485
Specification
MIKROLATEST® BP
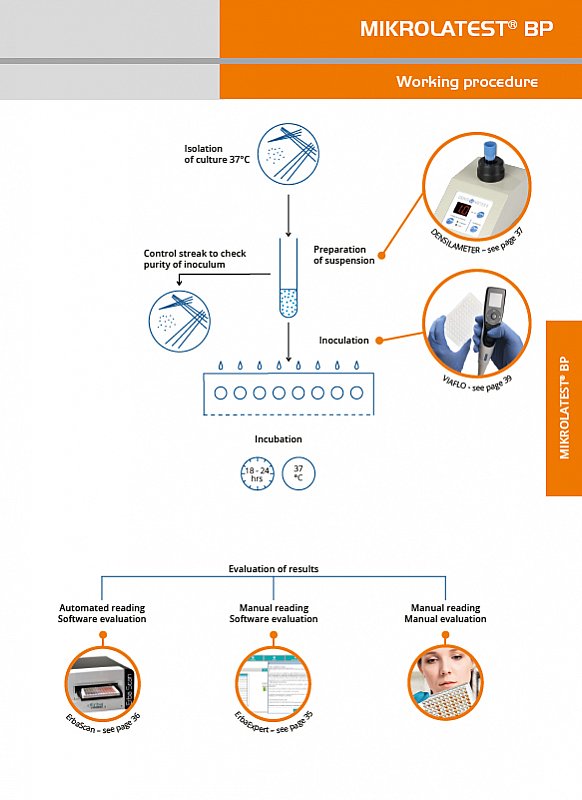
MIKROLATEST® BP kits are antibiotic susceptibility testing (AST) panels based on bacterial growth in breakpoint concentrations of defined antibiotics. Breakpoints refer to standards of European Committee for Antimicrobial Susceptibility Testing – EUCAST issued in January 2011. Most of the antibiotics are represented by two (in exceptional cases one or three) breakpoint concentrations which enable interpretation to three categories: sensitive, intermediate or resistant.
These categories are characterised by EUCAST* as follows:
Microorganism is defined as sensitive by a level of antimicrobial activity associated with a high probability of therapeutic success, Microorganism is defined as intermediate by a level of antimicrobial activity associated with uncertain therapeutic effect, Microorganism is defined as resistant by a level of antimicrobial activity associated with a high probability of therapeutic failure.
In each test system there is a growth control.
Similar Products
You might also be interested in these products